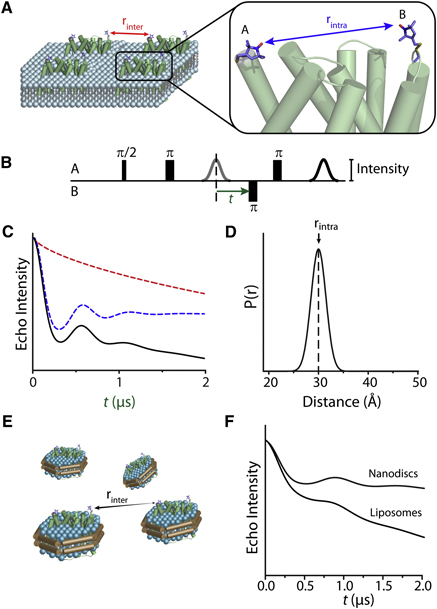
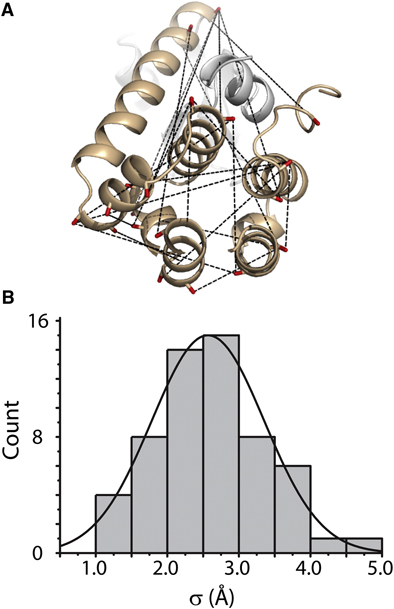
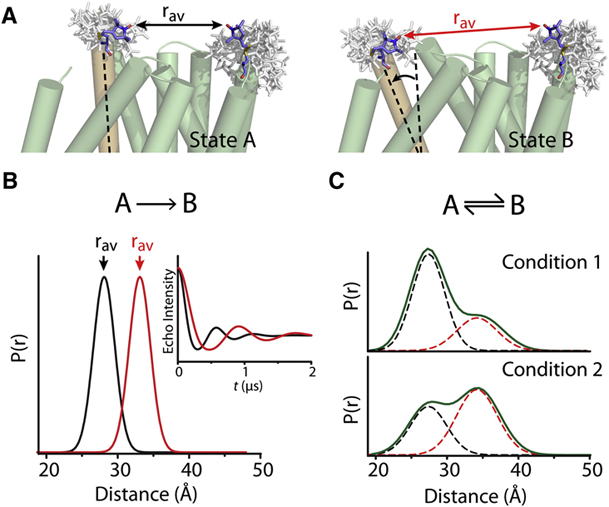
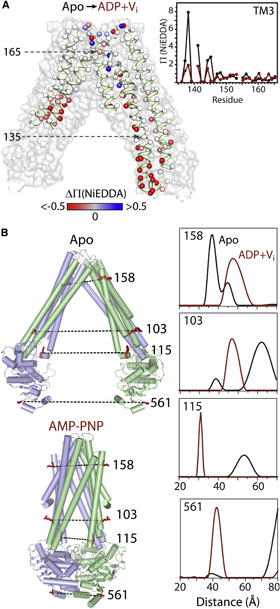
Toward the fourth dimension of membrane protein structure: Insight into dynamics from spin-labeling EPR spectroscopy
By Hassane Mchaourab, P. Ryan Steed, and Kelli Kazmier.
Published in Structure 19(11): 1549-61 on November 9, 2011.
PMID: 22078555. PMCID: PMC3224804. Link to Pubmed page.
Project: Structural Dynamics of ABC Transporter
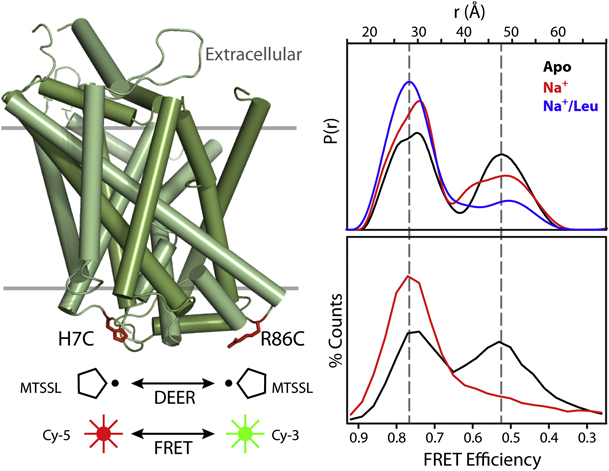
Figure 6. Conformational Fluctuations of LeuT Intracellular Side Revealed by DEER and FRET. Cys at positions 7 and 86 on the intracellular side of LeuT were modified with the Cy-5/Cy-3 FRET pair or with spin labels. Distance distributions from DEER (upper panel) or FRET efficiency histograms from single-molecule FRET (lower panel) were measured in the apo (black), Na+-bound (red), and Na+/Leu-bound (blue) states.
Abstract
Trapping membrane proteins in the confines of a crystal lattice obscures dynamic modes essential for interconversion between multiple conformations in the functional cycle. Moreover, lattice forces could conspire with detergent solubilization to stabilize a minor conformer in an ensemble thus confounding mechanistic interpretation. Spin labeling in conjunction with electron paramagnetic resonance (EPR) spectroscopy offers an exquisite window into membrane protein dynamics in the native-like environment of a lipid bilayer. Systematic application of spin labeling and EPR identifies sequence-specific secondary structures, defines their topology and their packing in the tertiary fold. Long range distance measurements (60 Å–80 Å) between pairs of spin labels enable quantitative analysis of equilibrium dynamics and triggered conformational changes. This review highlights the contribution of spin labeling to bridging structure and mechanism. Efforts to develop methods for determining structures from EPR restraints and to increase sensitivity and throughput promise to expand spin labeling applications in membrane protein structural biology.