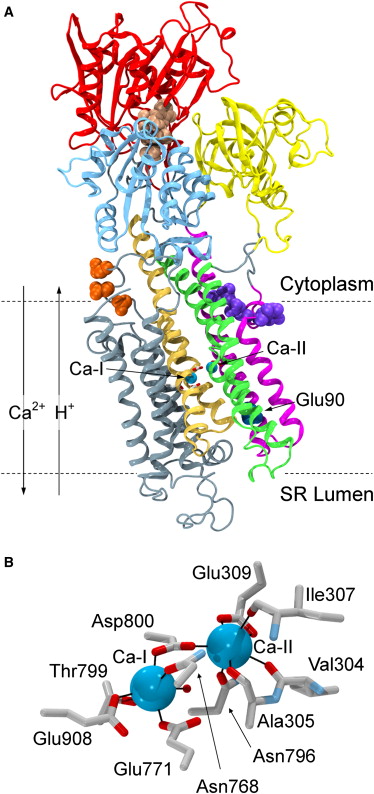
Tracing cytoplasmic Ca2+ ion and water access points in the Ca2+-ATPase
By Maria Musgaard, Lea Thøgersen, Birgit Schiøtt, and Emad Tajkhorshid.
Published in Biophysical Journal 102(2): 268-277 on January 18, 2012.
PMID: 22339863. PMCID: PMC3260667. Link to Pubmed page.
Core Facility: Computational Modeling
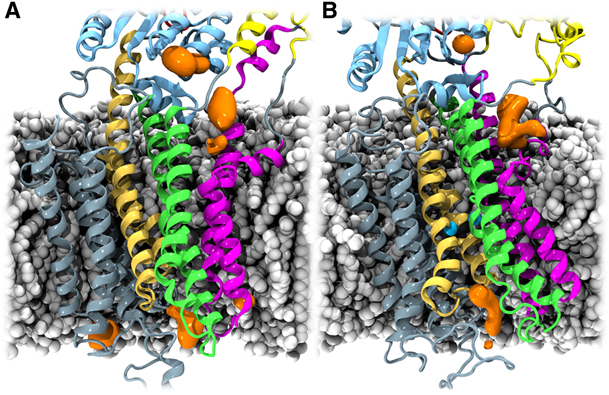
Figure 1. Cation-attracting regions of SERCA. The POPC molecules, protein, and ions are illustrated as in Figure 1 and Figure 2. K+ ion densities, calculated with the VolMap plug-in of VMD (27), are shown in orange. For both the Ca2+-free (A) and Ca2+-bound (B) states, K+ ions accumulate in the region between the kinked part of TM1 and TM2 (density in the cytoplasmic membrane-water interface), around the crystallographically determined K+-binding site (22) in the P domain (topmost density in the figures), and along the putative luminal exit path for Ca2+ ions (9).
Abstract
Sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA) transports two Ca2+ ions across the membrane of the sarco(endo)plasmic reticulum against the concentration gradient, harvesting the required energy by hydrolyzing one ATP molecule during each transport cycle. Although SERCA is one of the best structurally characterized membrane transporters, it is still largely unknown how the transported Ca2+ ions reach their transmembrane binding sites in SERCA from the cytoplasmic side. Here, we performed extended all-atom molecular dynamics simulations of SERCA. The calculated electrostatic potential of the protein reveals a putative mechanism by which cations may be attracted to and bind to the Ca2+-free state of the transporter. Additional molecular dynamics simulations performed on a Ca2+-bound state of SERCA reveal a water-filled pathway that may be used by the Ca2+ ions to reach their buried binding sites from the cytoplasm. Finally, several residues that are involved in attracting and guiding the cations toward the possible entry channel are identified. The results point to a single Ca2+ entry site close to the kinked part of the first transmembrane helix, in a region loaded with negatively charged residues. From this point, a water pathway outlines a putative Ca2+ translocation pathway toward the transmembrane ion-binding sites.