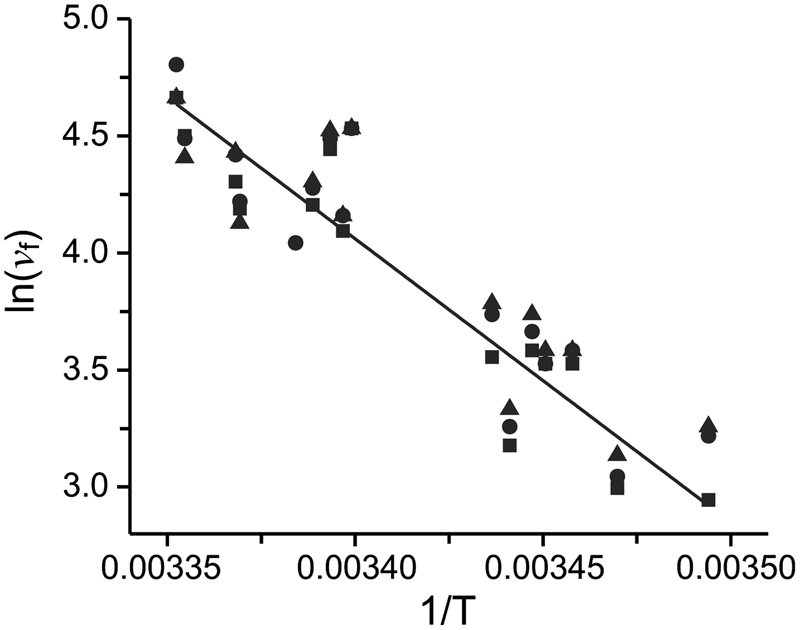
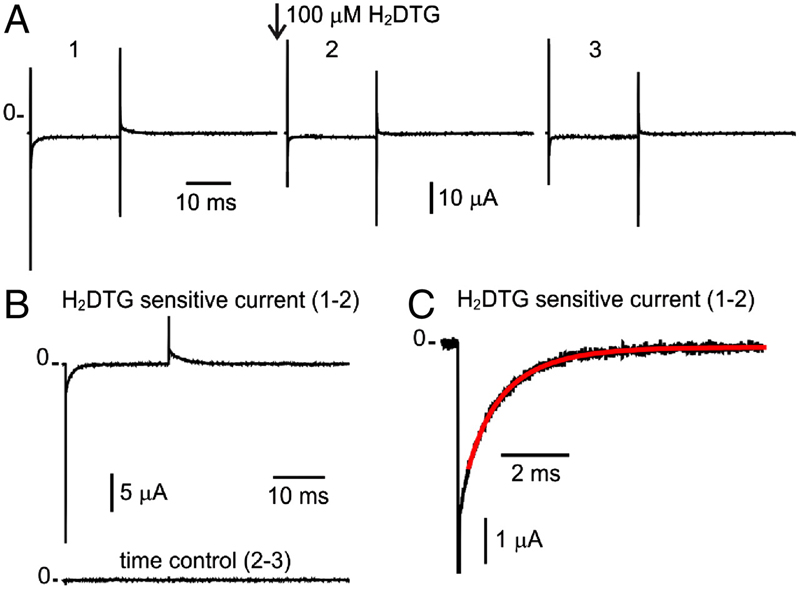
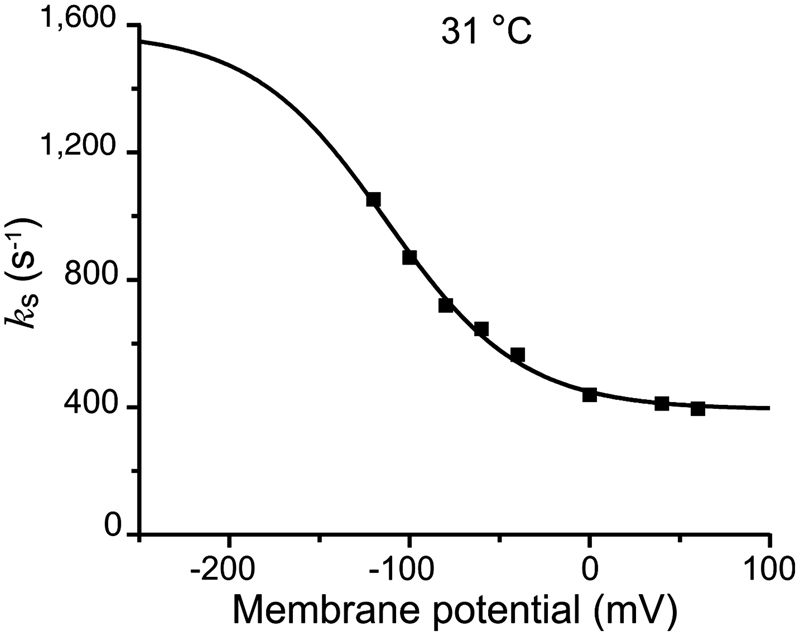
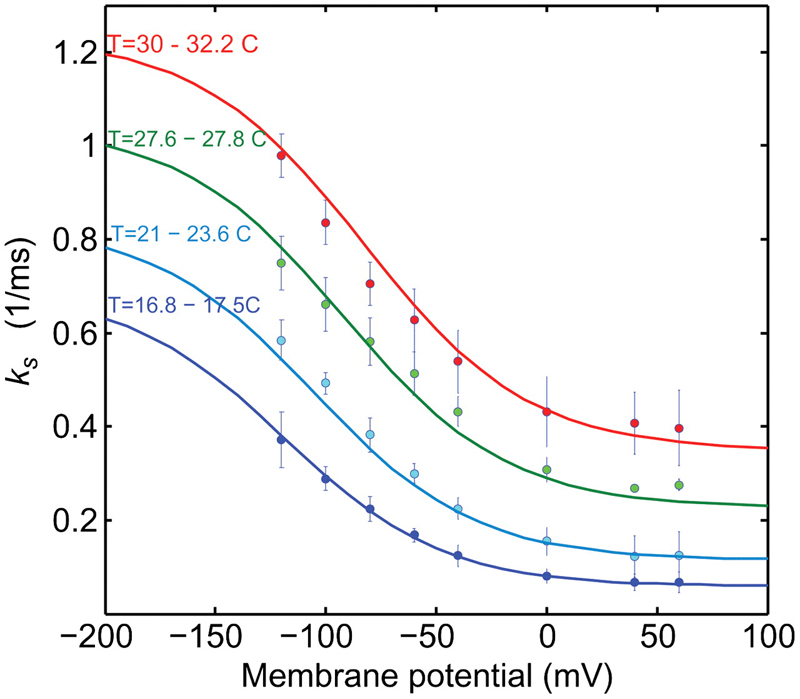
Energy landscape of the reactions governing the Na+ deeply occluded state of the Na+/K+-ATPase in the giant axon of the Humboldt squid
By Juan P. Castillo, Daniela De Giorgis, Daniel Basilio, David C. Gadsby, Joshua J. C. Rosenthal, Ramon Latorre, Miguel Holmgren, and Francisco Bezanilla.
Published in Proceedings of the National Academy of Sciences of the United States of America 108(51): 20556-61 on December 20, 2011.
PMID: 22143771. PMCID: PMC3251152. Link to Pubmed page.
Project: Conformational Transitions in P-class ATPases
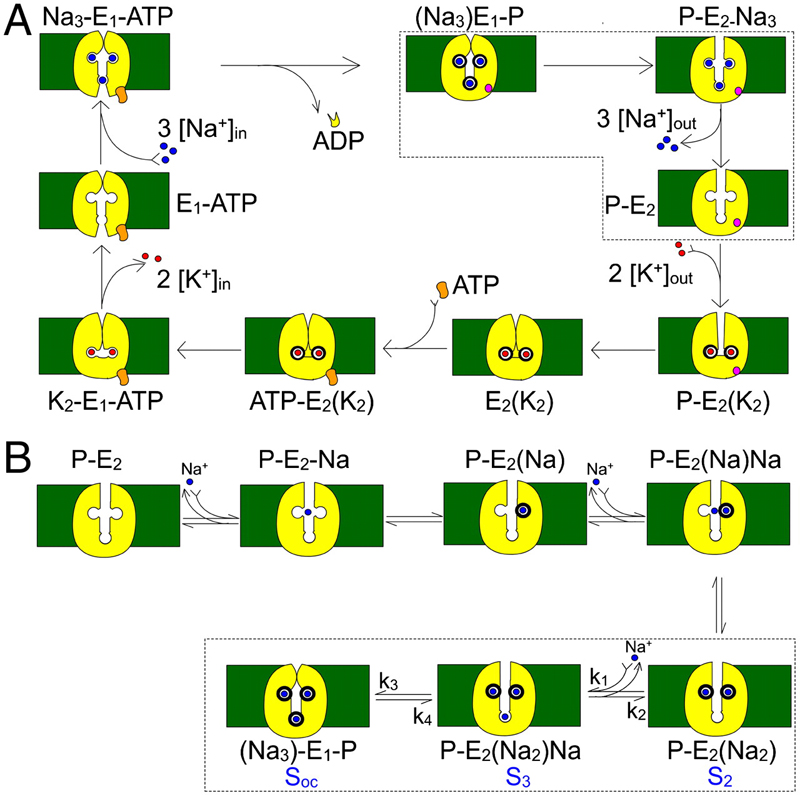
Figure 1. Kinetic schemes. (A) Simplified Albers-Post model for the Na+/K+ pump cycle. E1 and E2 are the main Na+/K+ pump conformations with ion binding sites facing the intracellular and extracellular sides, respectively. Internal (0 ADP, 0 P, 0 K+, and an ATP regenerating system) and external solutions (0 Na+) were designed to force the pump to operate in a strictly forward direction (Fig. 3). (B) Explicit extracellular Na+ binding/release and occlusion/deocclusion model. Electrogenic binding and release of each Na+ is assumed to be independent and in strict sequence. Similarly, Na+ are occluded and deoccluded independently but with electroneutral transitions. States encircled by dotted lines represent the binding/release and occlusion/deocclusion governing the slow component of the transient currents. Here we have assumed that the deep occlusion and the E1-P↔E2-P transitions are the same reaction.
Abstract
The Na(+)/K(+) pump is a nearly ubiquitous membrane protein in animal cells that uses the free energy of ATP hydrolysis to alternatively export 3Na(+) from the cell and import 2K(+) per cycle. This exchange of ions produces a steady-state outwardly directed current, which is proportional in magnitude to the turnover rate. Under certain ionic conditions, a sudden voltage jump generates temporally distinct transient currents mediated by the Na(+)/K(+) pump that represent the kinetics of extracellular Na(+) binding/release and Na(+) occlusion/deocclusion transitions. For many years, these events have escaped a proper thermodynamic treatment due to the relatively small electrical signal. Here, taking the advantages offered by the large diameter of the axons from the squid Dosidicus gigas, we have been able to separate the kinetic components of the transient currents in an extended temperature range and thus characterize the energetic landscape of the pump cycle and those transitions associated with the extracellular release of the first Na(+) from the deeply occluded state. Occlusion/deocclusion transition involves large changes in enthalpy and entropy as the ion is exposed to the external milieu for release. Binding/unbinding is substantially less costly, yet larger than predicted for the energetic cost of an ion diffusing through a permeation pathway, which suggests that ion binding/unbinding must involve amino acid side-chain rearrangements at the site.