On the Origin of Large Flexibility of P-glycoprotein in the Inward-Facing State
By Po-Chao Wen, Brandy Verhalen, Stephen Wilkens, Hassane Mchaourab and Emad Tajkhorshid.
Published in Journal of Biological Chemistry May 8, 2013;288(26): 19211-20. PMID: 23658020. PMCID: PMC3974643. Link to publication page.
Project: Structural Dynamics of ABC Transporter. Core Facility: Computational Modeling.
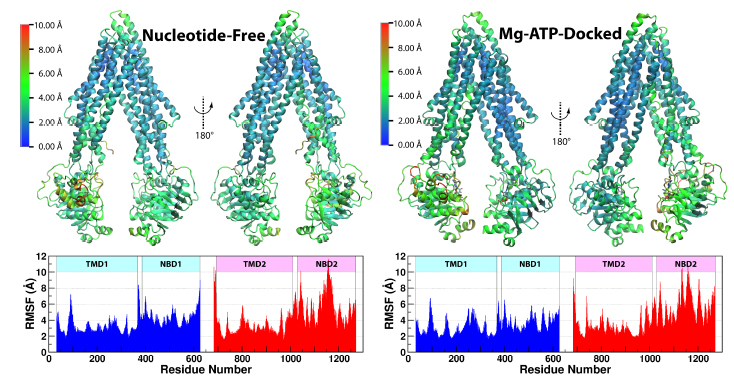
Figure 3. The RMSF of Ca atoms of Pgp under each of the two simulating conditions. RMSF was calculated for all four systems combined and shown as a heat map over a representative structure. The RMSF results are not significantly alatered after excluding the first 10 ns of trajectories of each system (seeFig. S7b).
Abstract
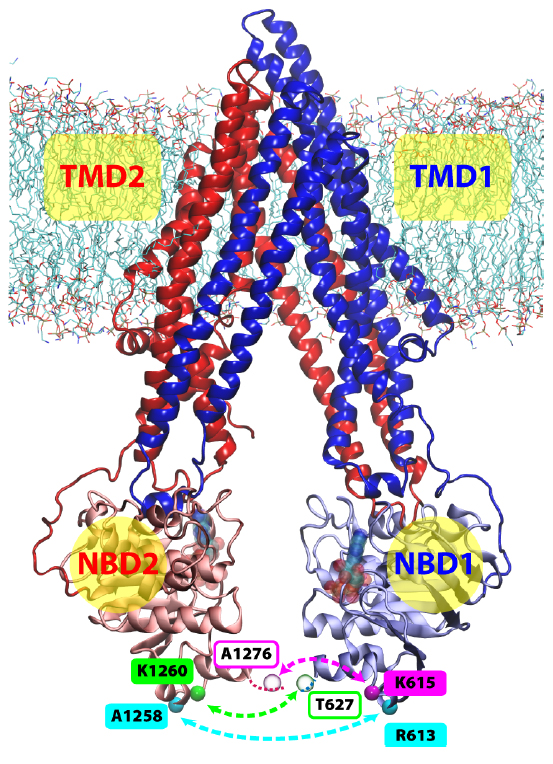
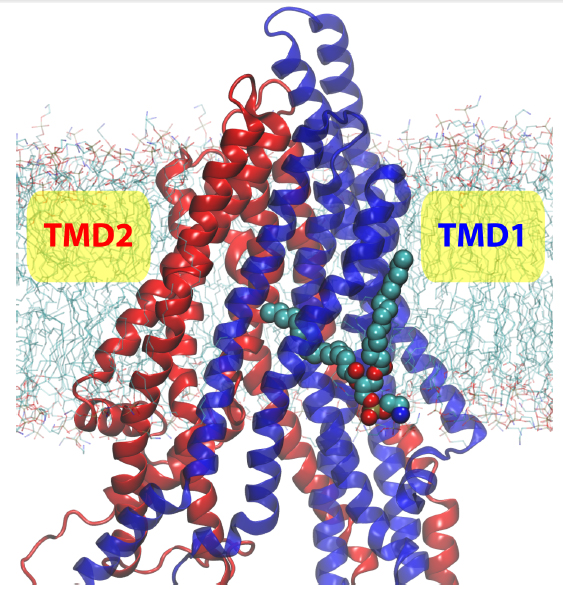
P-glycoprotein is one of the most biomedically relevant transporters in the ATP-binding cassette (ABC) superfamily, due to its involvement in developing multidrug resistance in cancer cells. Employing molecular dynamics simulations and double electron-electron resonance (DEER) spectroscopy, we have investigated the structural dynamics of membrane-bound Pgp in the inward-facing state and found that Pgp adopts an unexpectedly wide range of conformations, highlighted by the degree of separation between the two nucleotide-binding domains (NBDs). The distance between the two NBDs in the equilibrium simulations covers a range of at least 20 A, including both more open and more closed NBD configurations than the crystal structure. The DEER measurements on spin-labeled Pgp mutants also show wide distributions covering both longer and shorter distances than those observed in the crystal structure. Based on structural and sequence analyses, we propose that the transmembrane domains of Pgp might be more flexible than other structurally known ABC exporters. The structural flexibility of Pgp demonstrated here is not only in close agreement with, but also help rationalize, the reported high NBD fluctuations in several ABC exporters, and is possibly representing a fundamental difference in the transport mechanism between ABC exporters and ABC importers. In addition, during the simulations we have captured partial entrance of a lipid molecule from the bilayer into the lumen of Pgp reaching the putative drug binding site. The location of the protruding lipid suggests a putative pathway for direct drug recruitment from the membrane.