The membrane protein LeuT in micellar systems: aggregation dynamics and detergent binding to the S2 site
By George Khelashvili, Michael V. LeVine, Lei Shi, Matthias Quick, Jonathan A. Javitch, and Harel Weinstein.
Published in Journal of the American Chemical Society, 135(38):14266-75 on September 25, 2013. PMID: 23980525. PMCID: PMC3788620. Link to publication page.
Project: The Transport Cycle in Neurotransmitter Uptake Systems. Core Facility: Computational Modeling.
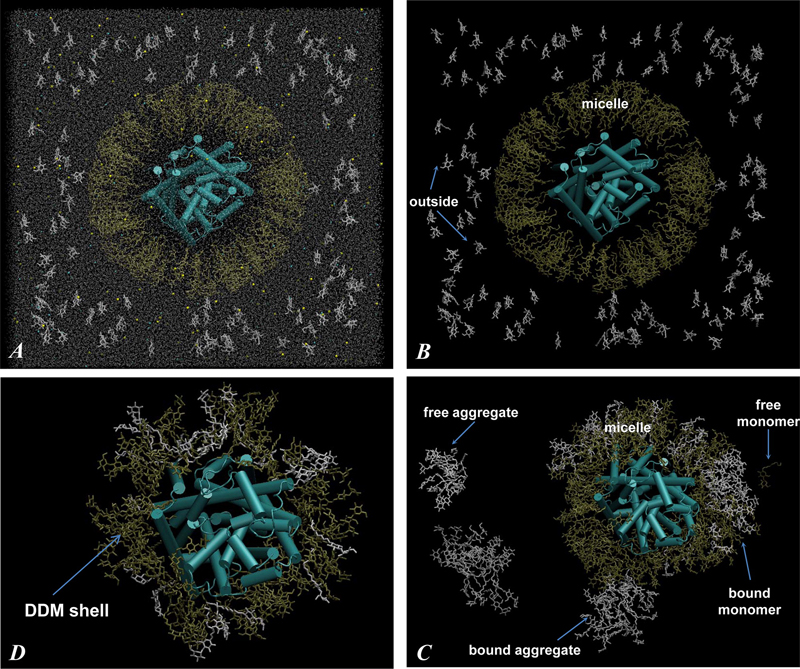
Figure 2. (A) Snapshot of the initial configuration of the 160/115 system (Figure 1, Starting Configurations). The cubic simulation unit box of 180 Å linear length contains LeuT protein (in cartoon), DDM detergent molecules (in licorice, 160 DDMs depicted in gold are in the micelle surrounding LeuT and 115 DDMs in white are in monomeric form outside), a water box (silver dots represent water oxygen atoms), and 0.15 M NaCl (yellow and cyan spheres). The leucine ligand bound to the LeuT S1 site and the two Na+ ions are omitted. (B) Same as in panel A only with water and salt ions removed. (C) Same as in panel B, only after 140 ns of MD simulations. Different types of aggregates (definitions in Methods) are highlighted with arrows and labeled. (D) Final snapshot of the 160/115 system, after 242 ns of simulations, showing only LeuT (cartoon) and shell DDM molecules (within 4 Å of protein) (licorice). Notice strong intermixing of initially micellar (gold) and monomeric (white) DDMs in panels C and D.
Abstract
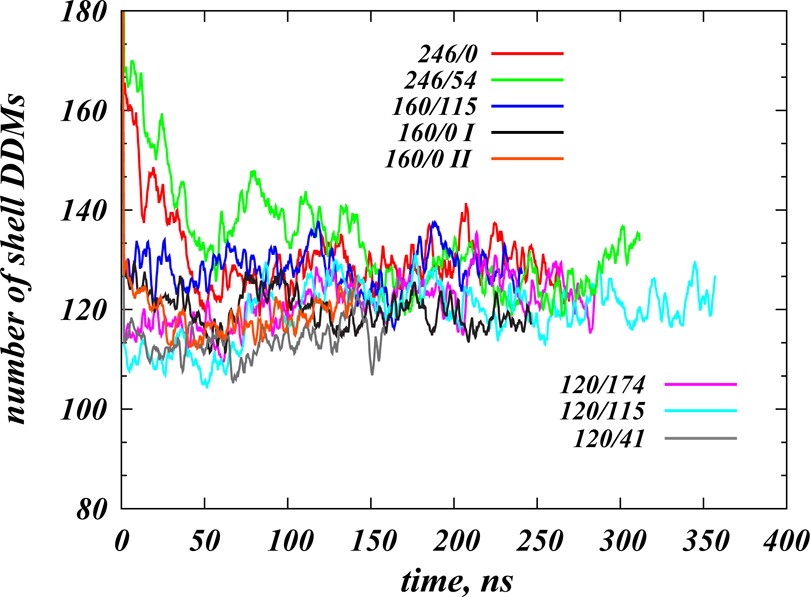
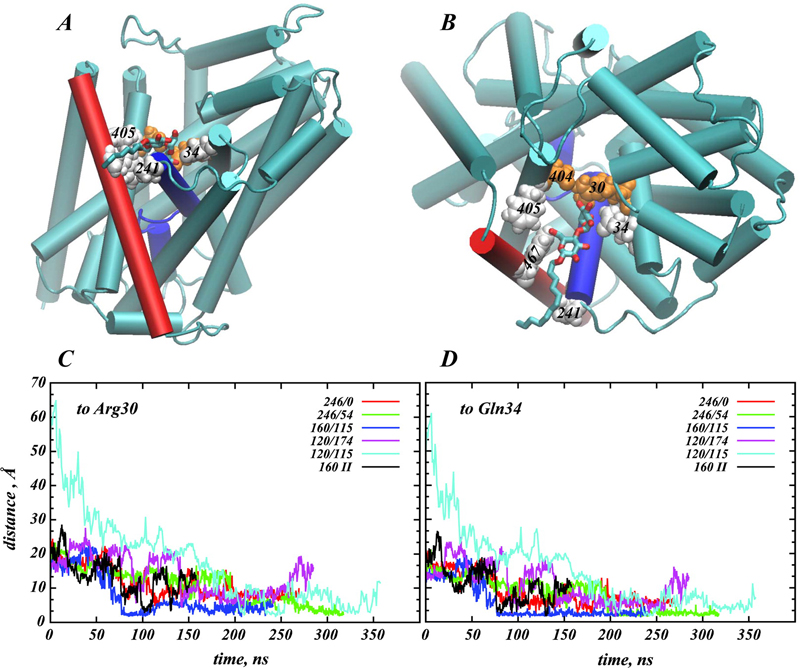
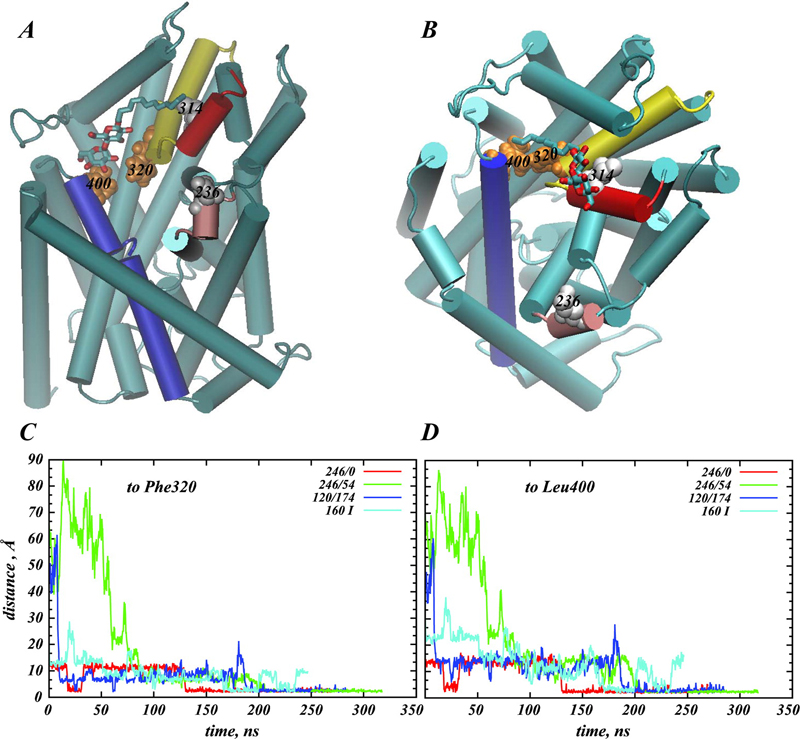
Structural and functional properties of integral membrane proteins are often studied in detergent micellar environments (proteomicelles), but how such proteomicelles form and organize is not well understood. This makes it difficult to evaluate the relationship between the properties of the proteins measured in such a detergent-solubilized form and under native conditions. To obtain mechanistic information about this relationship for the leucine transporter (LeuT), a prokaryotic homologue of the mammalian neurotransmitter/sodium symporters (NSSs), we studied the properties of proteomicelles formed by n-dodecyl-β,d-maltopyranoside (DDM) detergent. Extensive atomistic molecular dynamics simulations of different protein/detergent/water number ratios revealed the formation of a proteomicelle characterized by a constant-sized shell of detergents surrounding LeuT protecting its transmembrane segments from unfavorable hydrophobic/hydrophilic exposure. Regardless of the DDM content in the simulated system, this shell consisted of a constant number of DDM molecules (120 measured at a 4 Å cutoff distance from LeuT). In contrast, the overall number of DDMs in the proteomicelle (aggregation number) was found to depend on the detergent concentration, reaching a saturation value of 226±17 DDMs in the highest concentration regime simulated. Remarkably, we found that at high detergent-to-protein ratios we observed two independent ways of DDM penetration into LeuT, both leading to a positioning of the DDM molecule in the second substrate (S2) binding site of LeuT. Consonant with several recent experimental studies demonstrating changes in functional properties of membrane proteins due to detergent, our findings highlight how the environment in which the membrane proteins are examined may affect the outcome and interpretation of their mechanistic features.