Conformational dynamics of ligand-dependent alternating access in LeuT
By Kelli Kazmier, Shruti Sharma, Matthias Quick, Shahidul M Islam, Benoît Roux, Harel Weinstein, Jonathan Javitch and Hassane Mchaourab.
Published in Nature Structural & Molecular Biology 2014 Apr 20;21(5):472-9. PMID: 24747939. PMCID: PMC4050370. Link to publication page.
Project: The Transport Cycle in Neurotransmitter Uptake Systems. Core Facility: Computational Modeling.
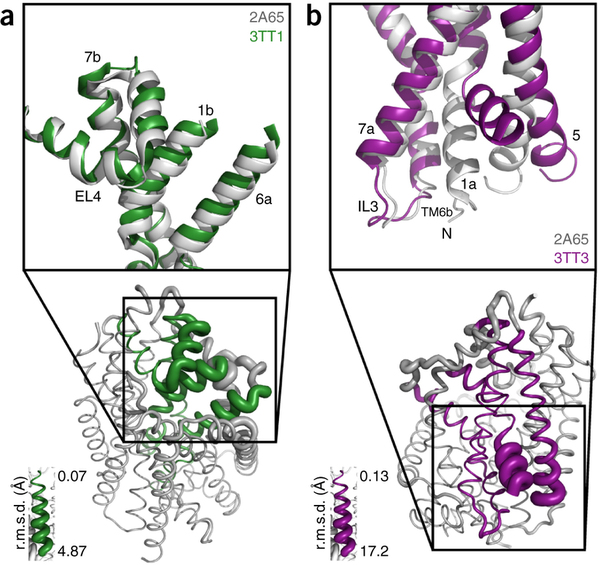
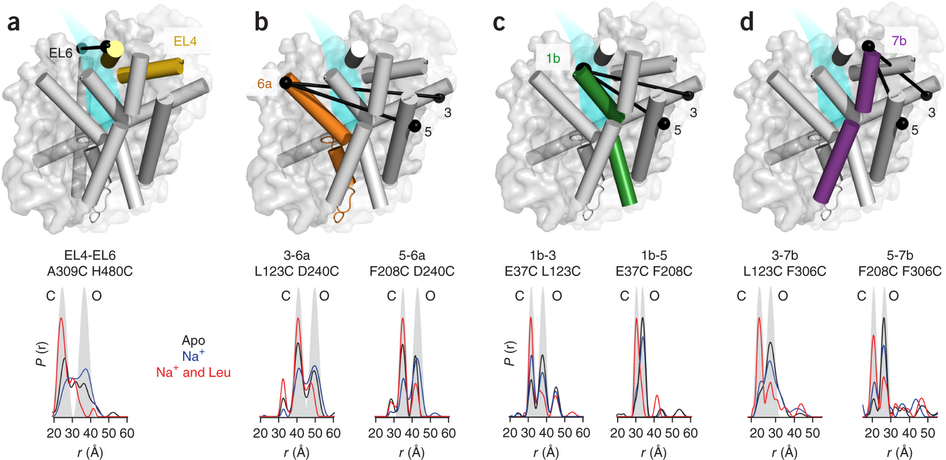
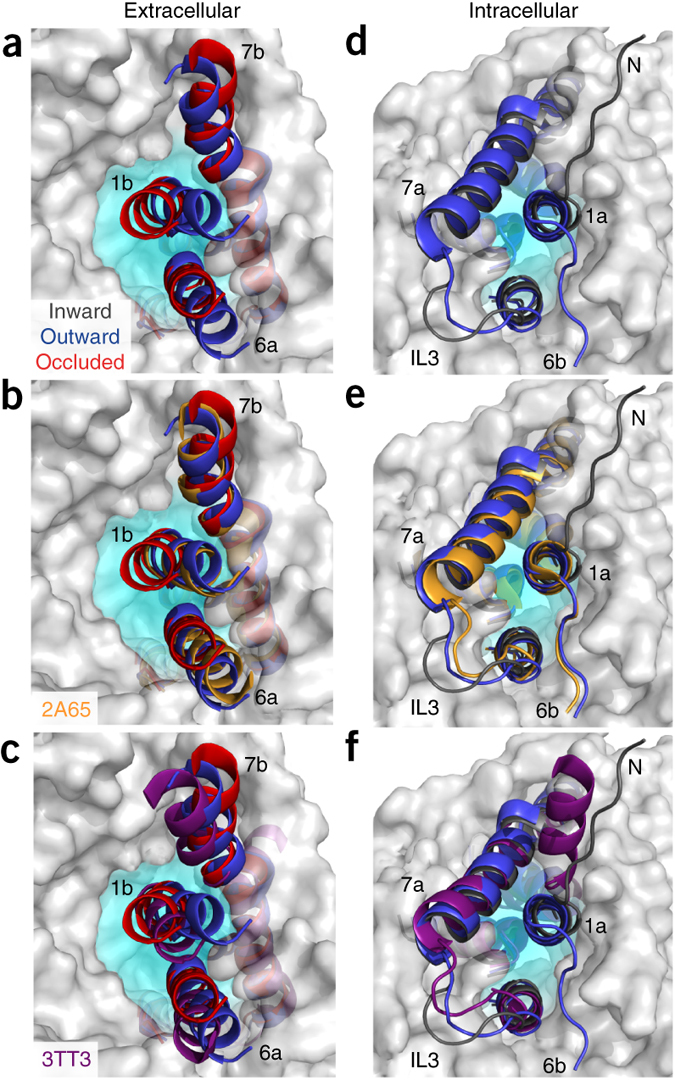
Figure 1. Model of LeuT alternating access inferred from the crystal structures. (a) r.m.s. deviations (r.m.s.d.) between occluded (PDB 2A65) and outward-open (PDB 3TT1) LeuT crystal structures mapped onto the outward-facing substrate-occluded structure. Inset, close-up view to highlight regions of most substantial differences: TMs 1 and 6, EL4 and TM7. (b) r.m.s. deviations between occluded (PDB 2A65) and inward-open (PDB 3TT3) LeuT crystal structures mapped onto a ribbon diagram of the outward-facing substrate-occluded structure. The regions of most appreciable differences (TMs 1 and 5) as well as TMs 6 and 7 are shown in a close-up view.
Abstract
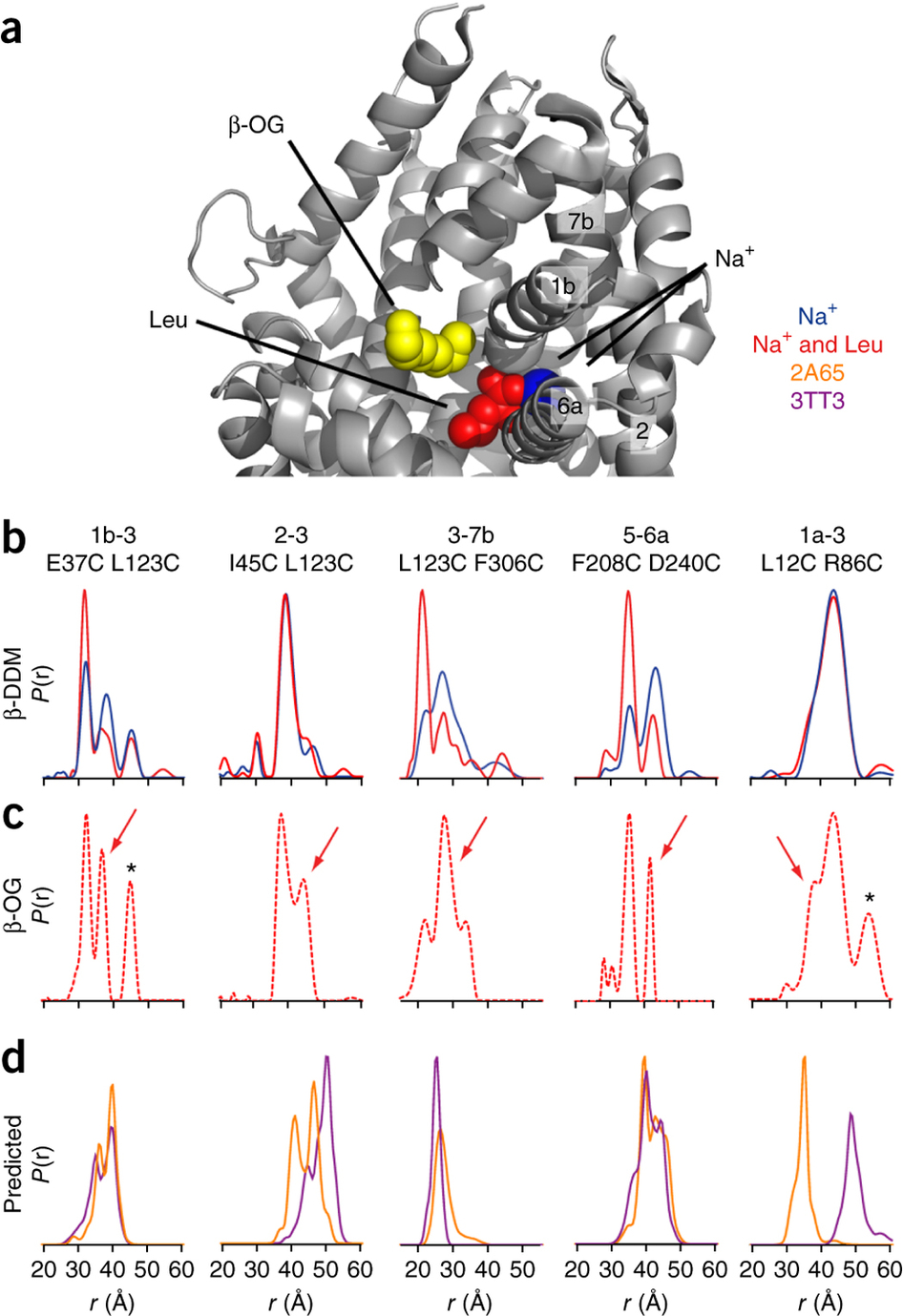
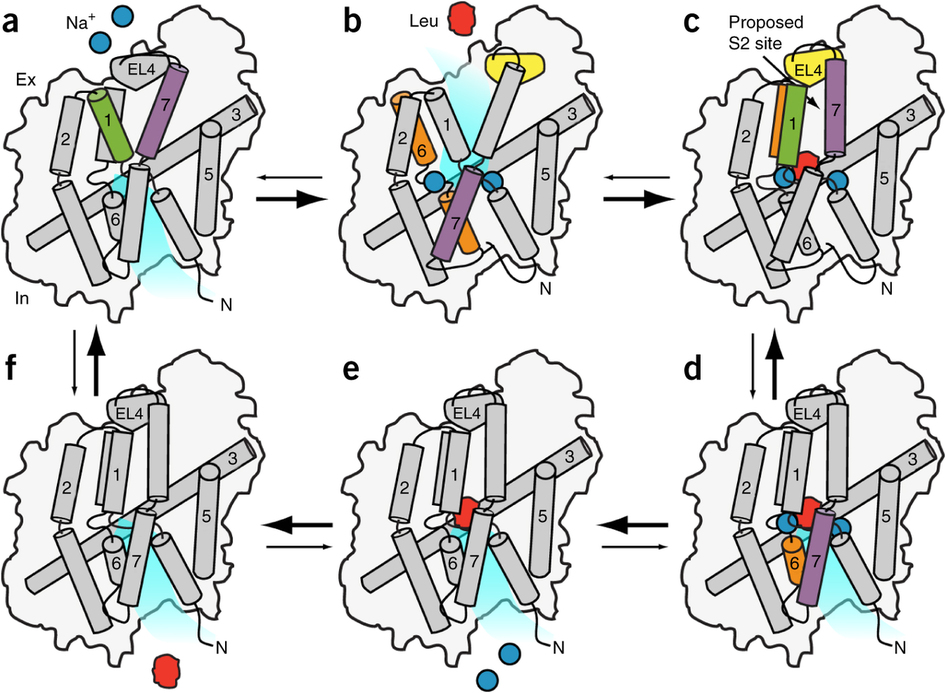
The leucine transporter (LeuT) from Aquifex aeolicus is a bacterial homolog of neurotransmitter/sodium symporters (NSSs) that catalyze reuptake of neurotransmitters at the synapse. Crystal structures of wild-type and mutants of LeuT have been interpreted as conformational states in the coupled transport cycle. However, the mechanistic identities inferred from these structures have not been validated, and the ligand-dependent conformational equilibrium of LeuT has not been defined. Here, we used distance measurements between spin-label pairs to elucidate Na+- and leucine-dependent conformational changes on the intracellular and extracellular sides of the transporter. The results identify structural motifs that underlie the isomerization of LeuT between outward-facing, inward-facing and occluded states. The conformational changes reported here present a dynamic picture of the alternating-access mechanism of LeuT and NSSs that is different from the inferences reached from currently available structural models.