Structural intermediates in a model of the substrate translocation path of the bacterial glutamate transporter homologue GltPh
By Sebastian Stolzenberg, George Khelashvili, and Harel Weinstein.
Published in J Phys Chem B. 2012 on May 10;116(18):5372-83.
PMID: 22494242. PMCID: PMC3350225. Link to publication page.
Core Facility: Computational Modeling
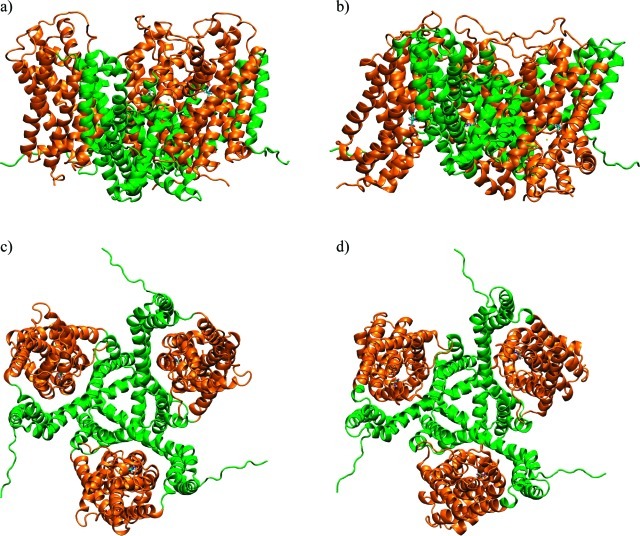
Figure 1. Crystal structures of GltPh. Outward-facing closed conformation (OfCC) (a and c) and inward-facing closed conformation (IfCC) (b and d), viewed from the side, parallel to the membrane (top) and from the top (bottom). Transport domains are represented as orange ribbons; trimerization domains are green. Sequence stretches in the N- and C-terminal loops that were unresolved in the crystal structures have been modeled using the program FUGUE, and parts of the TM3–4 loop, unresolved in the OfCC, were modeled with ArchPRED,32 as described in Methods.
Abstract
Excitatory amino acid transporters (EAATs) are membrane proteins responsible for reuptake of glutamate from the synaptic cleft to terminate neurotransmission and help prevent neurotoxically high, extracellular glutamate concentrations. Important structural information about these proteins emerged from crystal structures of GltPh, a bacterial homologue of EAATs, in conformations facing outward and inward. These remarkably different conformations are considered to be end points of the substrate translocation path (STP), suggesting that the transport mechanism involves major conformational rearrangements that remain uncharted. To investigate possible steps in the structural transitions of the STP between the two end-point conformations, we applied a combination of computational modeling methods (motion planning, molecular dynamics simulations, and mixed elastic network models). We found that the conformational changes in the transition involve mainly the repositioning the “transport domain” and the “trimerization domain” identified previously in the crystal structures. The two domains move in opposite directions along the membrane normal, and the transport domain also tilts by ∼17° with respect to this axis. Moreover, the TM3–4 loop undergoes a flexible, “restraining bar”-like conformational change with respect to the transport domain. As a consequence of these conformational rearrangements along the transition path we calculated a significant decrease of nearly 20% in the area of the transport-to-trimerization domain interface (TTDI). Water penetrates parts of the TTDI in the modeled intermediates but very much less in the end-point conformations. We show that these characteristics of the modeled intermediate states agree with experimental results from residue-accessibility studies in individual monomers and identify specific residues that can be used to test the proposed STP. Moreover, MD simulations of complete GltPh trimers constructed from initially identical monomer intermediates suggest that asymmetry can appear in the trimer, consonant with available experimental data showing independent transport kinetics by individual monomers in the trimers.


