Hydrogen bonds as molecular timers for slow inactivation in voltage-gated potassium channels
By Stephan A Pless, Jason D Galpin, Ana P Niciforovic, Harley T Kurata, and Christopher A Ahern.
Published in Elife 2013 Dec 10;2:e01289.
PMID: 24327560. PMCID: PMC3852034. Link to Pubmed page.
Core Facility: Membrane Protein Expression/Purification.
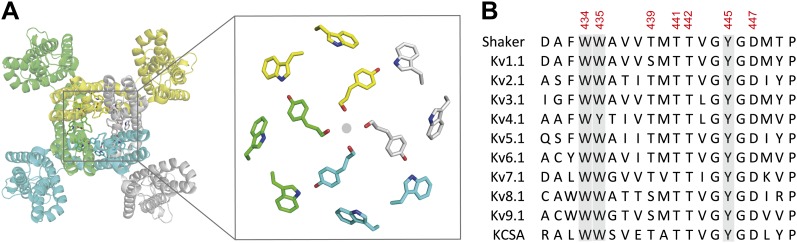
Figure 7. The aromatic cuff is part of a highly conserved region in potassium channels. (A) Top view of a Kv channel based on the structure of the tetrameric Kv1.2/2.1 chimera (PDB 2R9R; individual subunits are colored in gray, cyan, green and yellow, respectively). The inset shows a magnified view of the side chains that form the ‘aromatic cuff’: Trp434, Trp435 and Tyr445 (by numbering in Shaker potassium channels). Note the backbone carbonyls are shown for Tyr445 to highlight their role in the coordination of potassium ions (gray circle); (B) Sequence alignment of the pore helix and the selectivity filter of various potassium channels: Shaker (GI:288442), Kv1.1 (GI:119395748), Kv2.1 (GI:84570020), Kv3.1 (GI:298603), Kv4.1 (GI:8272404), Kv5.1 (GI:24418476), Kv6.1 (GI:24418479), Kv7.1 (GI:6166005), Kv8.1 (GI:7657289), Kv9.1 (GI:219520418), and KCSA (GI: 61226909). Side chains constituting the aromatic cuff are highlighted in gray (see above) and all positions studied here are indicated using their numbering in Shaker potassium channels (these residues correspond to Trp362, Trp363, Ser367, Thr369, Thr370, Tyr373 and Asp375 in the Kv1.2/Kv2.1 [voltage-gated potassium channel isoforms 1.2 and 2.1] chimera crystal structure [PDB 2R9R]).
Abstract
Voltage-gated potassium (Kv) channels enable potassium efflux and membrane repolarization in excitable tissues. Many Kv channels undergo a progressive loss of ion conductance in the presence of a prolonged voltage stimulus, termed slow inactivation, but the atomic determinants that regulate the kinetics of this process remain obscure. Using a combination of synthetic amino acid analogs and concatenated channel subunits we establish two H-bonds near the extracellular surface of the channel that endow Kv channels with a mechanism to time the entry into slow inactivation: an intra-subunit H-bond between Asp447 and Trp434 and an inter-subunit H-bond connecting Tyr445 to Thr439. Breaking of either interaction triggers slow inactivation by means of a local disruption in the selectivity filter, while severing the Tyr445–Thr439 H-bond is likely to communicate this conformational change to the adjacent subunit(s).


