Total chemical synthesis of biologically active fluorescent dye-labeled Ts1 toxin
By Bobo Dang, Tomoya Kubota, Ana M. Correa, Francisco Bezanilla and Stephen Kent.
Published in Angew Chem Int Ed Engl. 2014 Aug 18;53(34):8970-4. PMID: 24989851. Link to publication page.
Core Facility: Computational Modeling.
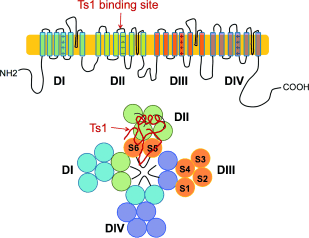
Figure 1. Cartoon representation of the α subunit of Nav. The Ts1 toxin protein molecule binds to the voltage-sensor domain (VSD) of DII; a hypothetical binding mode of Ts1 is shown.
Abstract
Ts1 toxin is a protein found in the venom of the Brazilian scorpion Tityus serrulatus. Ts1 binds to the domain II voltage sensor in the voltage-gated sodium channel Nav and modifies its voltage dependence. In the work reported here, we established an efficient total chemical synthesis of the Ts1 protein using modern chemical ligation methods and demonstrated that it was fully active in modifying the voltage dependence of the rat skeletal muscle voltage-gated sodium channel rNav1.4 expressed in oocytes. Total synthesis combined with click chemistry was used to label the Ts1 protein molecule with the fluorescent dyes Alexa-Fluor 488 and Bodipy. Dye-labeled Ts1 proteins retained their optical properties and bound to and modified the voltage dependence of the sodium channel Nav. Because of the highly specific binding of Ts1 toxin to Nav, successful chemical synthesis and labeling of Ts1 toxin provides an important tool for biophysical studies, histochemical studies, and opto-pharmacological studies of the Nav protein.


