Room-Temperature Distance Measurements of Immobilized Spin-Labeled Protein by DEER/PELDOR
By Virginia Meyer, Michael A. Swanson, Laura J. Clouston, Przemysław J. Boratyński, Richard A. Stein, Hassane Mchaourab, Andrzej Rajca, Sandra S. Eaton, and Gareth R. Eaton.
Published in Biophys J. 2015 Mar 10;108(5):1213-9.
PMID: 25762332. PMCID: 4375670. Link to publication page.
Core Facility: Membrane Protein Expression and Purification
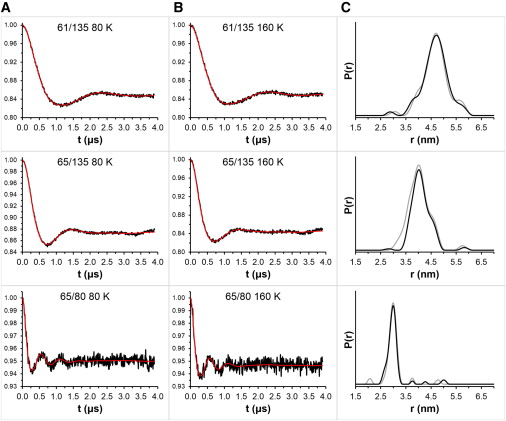
Figure 4.
Background-subtracted DEER data at 80 K (A, black) and 160 K (B, black) for T4L labeled with 3 in 24% glycerol. Tikhonov fitting is shown in red. The distance distributions (C) were similar at 80 K (black) and 160 K (gray) for each double mutant.
Abstract
Nitroxide spin labels are used for double electron-electron resonance (DEER) measurements of distances between sites in biomolecules. Rotation of gem-dimethyls in commonly used nitroxides causes spin echo dephasing times (Tm) to be too short to perform DEER measurements at temperatures between ∼80 and 295 K, even in immobilized samples. A spirocyclohexyl spin label has been prepared that has longer Tm between 80 and 295 K in immobilized samples than conventional labels. Two of the spirocyclohexyl labels were attached to sites on T4 lysozyme introduced by site-directed spin labeling. Interspin distances up to ∼4 nm were measured by DEER at temperatures up to 160 K in water/glycerol glasses. In a glassy trehalose matrix the Tm for the doubly labeled T4 lysozyme was long enough to measure an interspin distance of 3.2 nm at 295 K, which could not be measured for the same protein labeled with the conventional 1-oxyl-2,2,5,5-tetramethyl-3-pyrroline-3-(methyl)methanethio-sulfonate label.


