Mechanism of the Association between Na+ Binding and Conformations at the Intracellular Gate in Neurotransmitter:Sodium Symporters
By Sebastian Stolzenberg, Matthias Quick, Chunfeng Zhao, Kamil Gotfryd, George Khelashvili, Ulrik Gether, Claus J. Loland, Jonathan Javitch, Sergei Noskov, Harel Weinstein, and Lei Shi.
Published in J Biol Chem. 2015 May 29; 290(22): 13992–14003. PMID: 25869126. PMCID: PMC4447972. Link to publication page.
Project: The Transport Cycle in Neurotransmitter Uptake Systems. Core Facility: Computational Modeling.
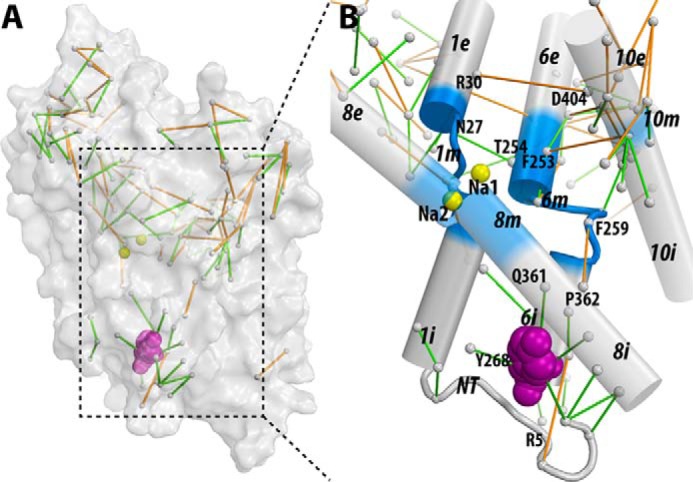
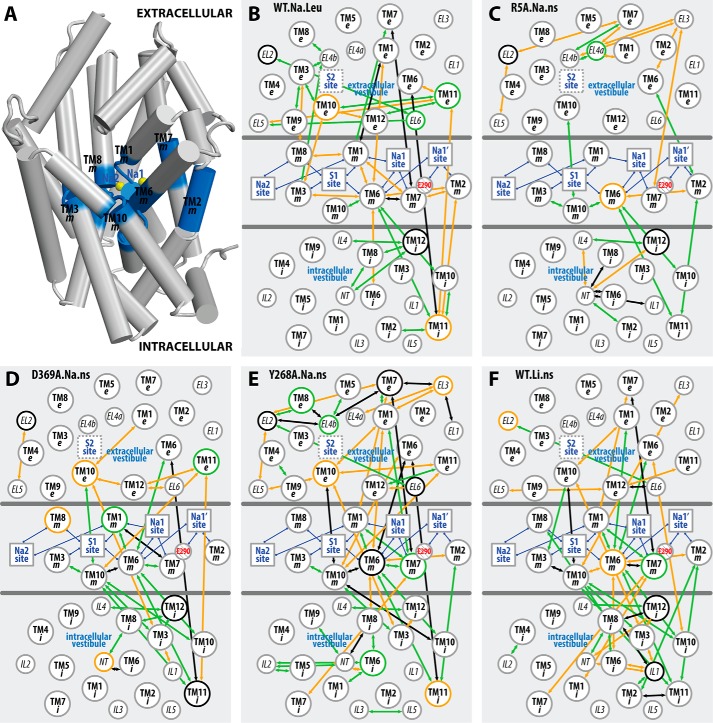
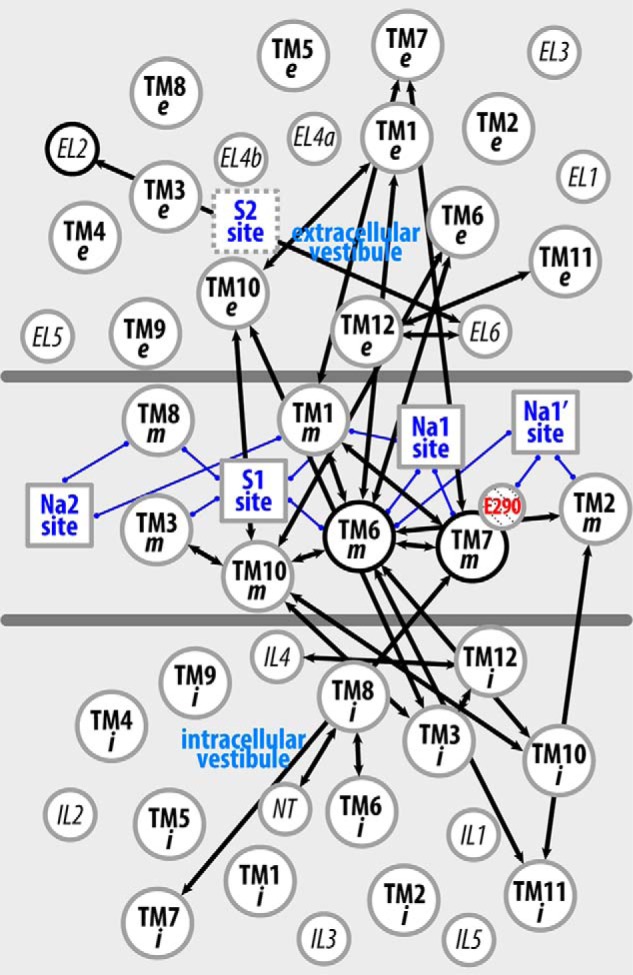
Figure 6. The interaction network that propagates the impact of the Y268A mutation from the intracellular gate to the substrate and Na+ binding sites. The pairwise residue interactions that are significantly more frequent in Y268A.Na.ns or WT.Na.ns are identified by orange or green lines, respectively. B, enlarged view of the marked area in A. Note that the pairwise residue interactions that were also affected by the R5A mutation (comparing R5A.Na.ns to WT.Na.ns) are probably less important for Na+-coupled transport and are not shown.
Abstract
Neurotransmitter:sodium symporters (NSSs) terminate neurotransmission by Na+-dependent reuptake of released neurotransmitters. Previous studies suggested that Na+-binding reconfigures dynamically coupled structural elements in an allosteric interaction network (AIN) responsible for function-related conformational changes, but the intramolecular pathway of this mechanism has remained uncharted. We describe a new approach for the modeling and analysis of intramolecular dynamics in the bacterial NSS homolog LeuT. From microsecond-scale molecular dynamics simulations and cognate experimental verifications in both LeuT and human dopamine transporter (hDAT), we apply the novel method to identify the composition and the dynamic properties of their conserved AIN. In LeuT, two different perturbations disrupting Na+ binding and transport (i.e. replacing Na+ with Li+ or the Y268A mutation at the intracellular gate) affect the AIN in strikingly similar ways. In contrast, other mutations that affect the intracellular gate (i.e. R5A and D369A) do not significantly impair Na+ cooperativity and transport. Our analysis shows these perturbations to have much lesser effects on the AIN, underscoring the sensitivity of this novel method to the mechanistic nature of the perturbation. Notably, this set of observations holds as well for hDAT, where the aligned Y335A, R60A, and D436A mutations also produce different impacts on Na+ dependence. Thus, the detailed AIN generated from our method is shown to connect Na+ binding with global conformational changes that are critical for the transport mechanism. That the AIN between the Na+ binding sites and the intracellular gate in bacterial LeuT resembles that in eukaryotic hDAT highlights the conservation of allosteric pathways underlying NSS function.