Architecture of the fungal nuclear pore inner ring complex
By Tobias Stuwe, Christopher J. Bley, Karsten Thierbach, Stefan Petrovic, Sandra Schilbach, Daniel J. Mayo, Thibaud Perriches, Emily J. Rundlet, Young E. Jeon, Leslie N. Collins, Ferdinand M. Huber, Daniel H. Lin, Marcin Paduch, Akiko Koide, Vincent Lu, Jessica Fischer, Ed Hurt, Shohei Koide, Anthony A. Kossiakoff and André Hoelz.
Published in Science. 2015 Oct 2;350(6256):56-64.
PMID: 26316600. Link to Pubmed page.
Core Facility: Synthetic Antigen Binder (SAB) Generation and Crystallography.
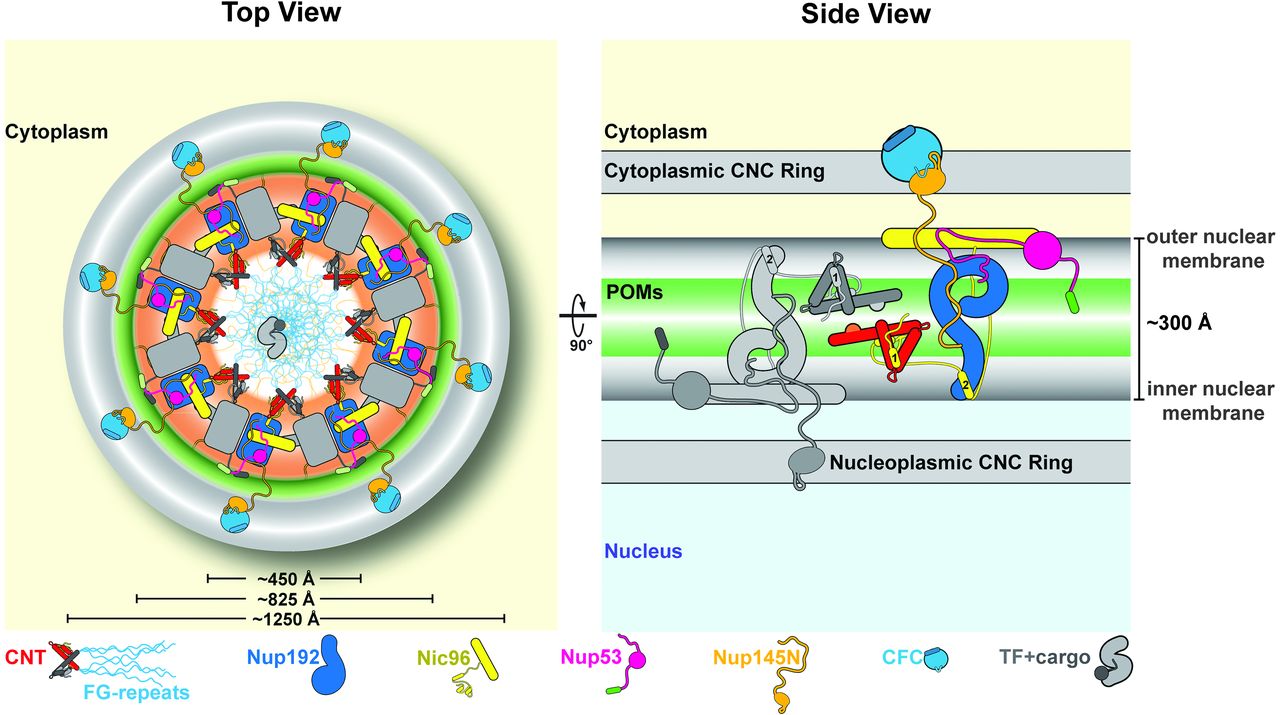
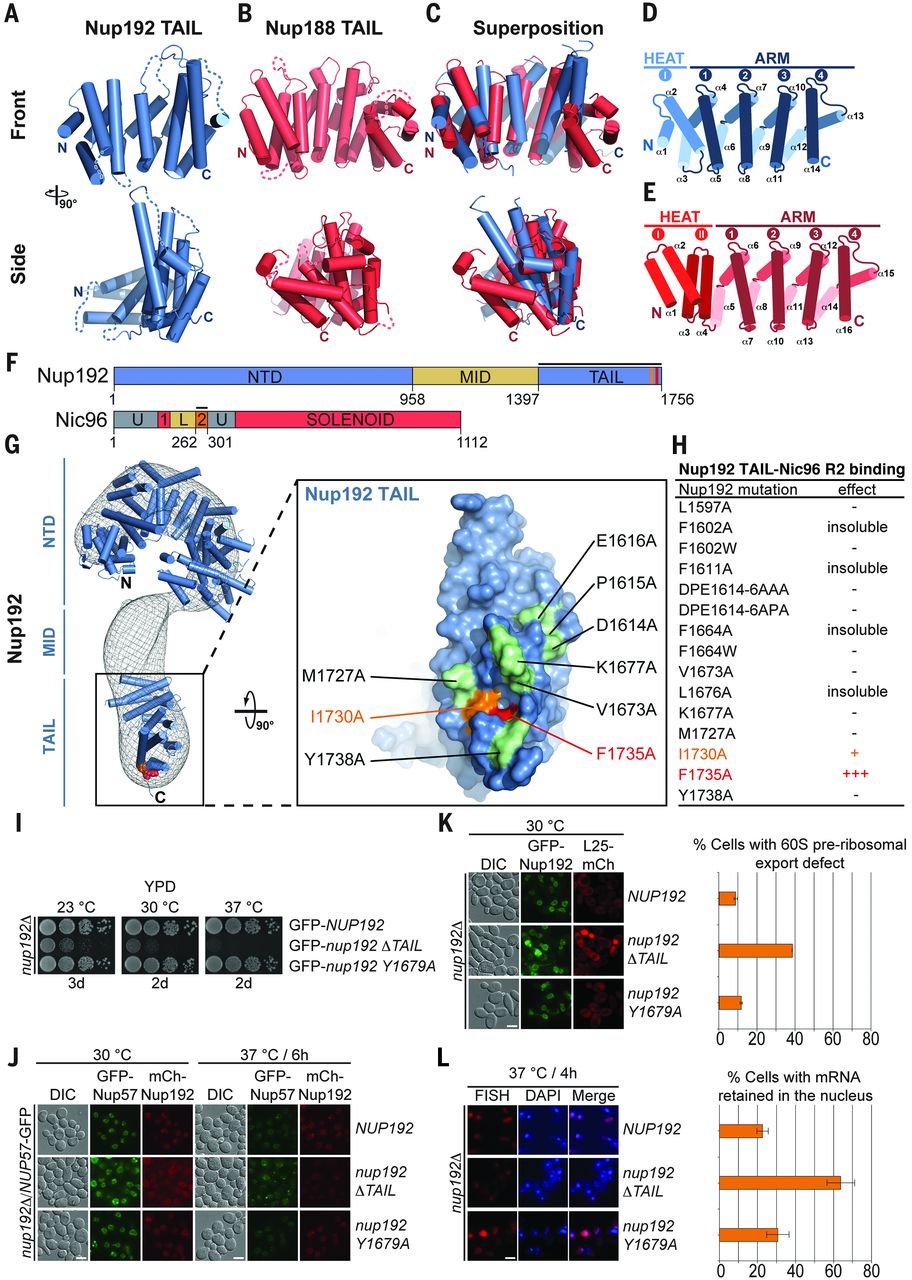
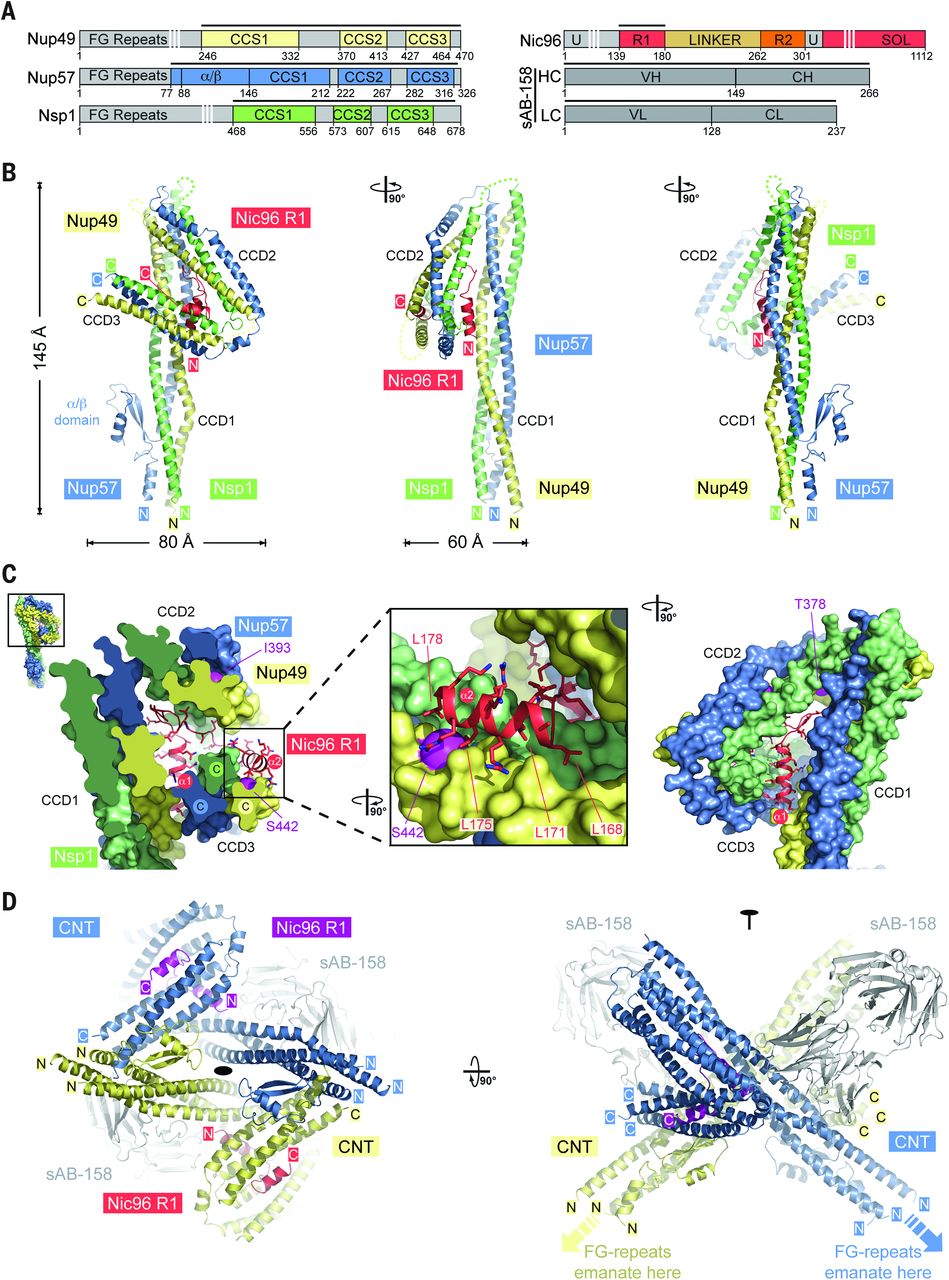
Figure 6. Proposed architecture of the NPC inner ring scaffold. Sixteen copies of the IRC are anchored to the nuclear pore membrane and arranged in a ring-shaped scaffold in an antiparallel fashion to satisfy the established eightfold and twofold symmetry axes of the NPC. Each IRC is composed of the channel nucleoporins Nsp1, Nup49, and Nup57 (CNT, red) and the adaptor nucleoporins Nup192 (blue), Nic96 (yellow), Nup53 (magenta), and Nup145N (orange). The cytoplasmic and nuclear CNC rings (gray), the putative location of the pore membrane proteins (POMs) (green), and the inner ring (red) are shown. On the cytoplasmic side, Nup145N attaches the cytoplasmic filament nucleoporins Nup82 and Nup159 (CFC, cyan). The primarily unstructured adaptor nucleoporins Nup53 and Nup145N mediate the association of various structured IRC components and thus are critical for the IRC scaffold integrity. Nic96 functions as an assembly sensor that recognizes the conformation of the overall 4-shaped three-stranded coiled-coil domain architecture of the CNT, thereby mediating the selective incorporation of a defined CNT state into the NPC. The CNTs are positioned in the equatorial plane of the inner ring, with the FG repeats of all three channel nups projecting toward the central transport channel. The antiparallel orientation of adjacent IRCs would generate two CNT rings, one at the top and another at the bottom of the central transport channel. This arrangement would allow for the formation of a transport factor–mediated FG repeat meshwork or hydrogel, which would further reinforce the inner ring, scaffold. For size reference, a β-karyopherin•cargo complex is shown in gray.
Abstract
The nuclear pore complex (NPC) constitutes the sole gateway for bidirectional nucleocytoplasmic transport. We present the reconstitution and interdisciplinary analyses of the ~425-kilodalton inner ring complex (IRC), which forms the central transport channel and diffusion barrier of the NPC, revealing its interaction network and equimolar stoichiometry. The Nsp1•Nup49•Nup57 channel nucleoporin heterotrimer (CNT) attaches to the IRC solely through the adaptor nucleoporin Nic96. The CNT•Nic96 structure reveals that Nic96 functions as an assembly sensor that recognizes the three-dimensional architecture of the CNT, thereby mediating the incorporation of a defined CNT state into the NPC. We propose that the IRC adopts a relatively rigid scaffold that recruits the CNT to primarily form the diffusion barrier of the NPC, rather than enabling channel dilation.
Editor’s Summary
Building a gate to the nucleus
Nuclear pore complexes form a gateway between the cytoplasm and the nucleus (see the Perspective by Ullman and Powers). Stuwe et al. combined structural, biochemical, and functional analyses to elucidate the architecture of a six-protein complex that makes up the inner ring of the fungal nuclear pore. This includes a central trimeric complex homologous to the Nup62 complex found in metazoans that is incorporated into the nuclear pore inner-ring complex. Chug et al. report the structure of the metazoan trimeric Nup62 complex. Neither study supports a model in which the pore can dilate and constrict. Instead they suggest a rigid pore in which flexible domains called FG repeats fill the channel and form a barrier that can be traversed by receptors that carry cargos across.