Crystal structures of a double-barrelled fluoride ion channel
By Randy B. Stockbridge, Ludmila Kolmakova-Partensky, Tania Shane, Akiko Koide, Shohei Koide, Christopher Miller, and Simon Newstead.
Published in Nature. 2015 Sep 24;525(7570):548-51.
PMID: 26344196. PMCID: 4876929. Link to Pubmed page.
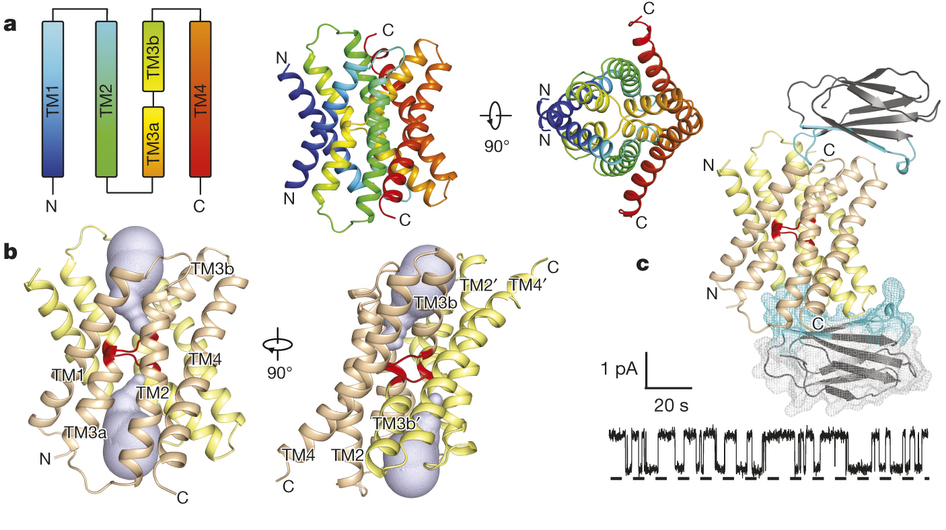
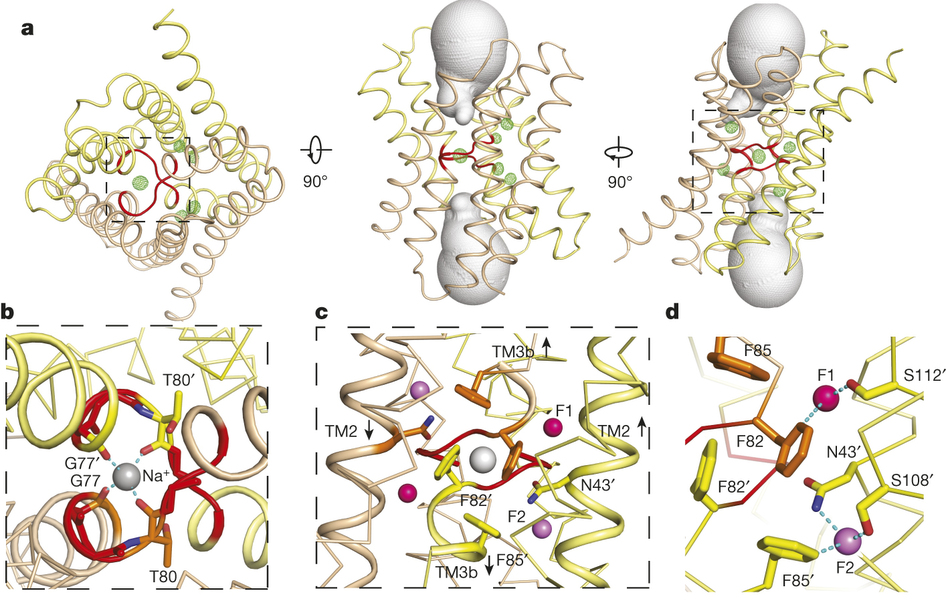
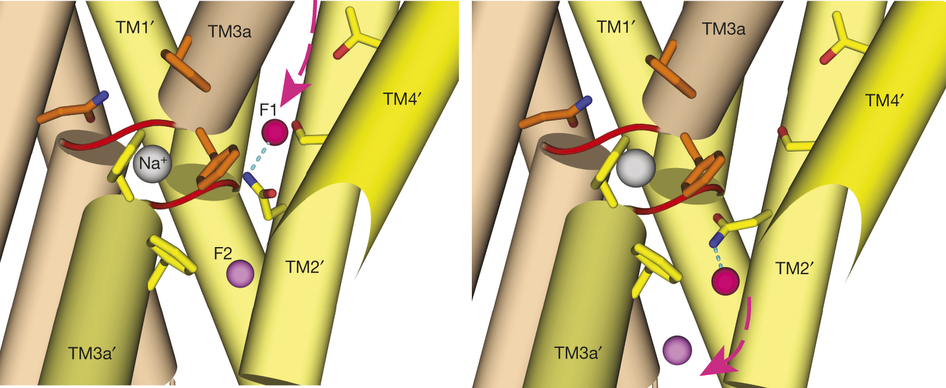
Figure 1. Bpe–S7 structure. a, Bpe homodimer, viewed parallel (centre structure) and perpendicular (right structure) to the membrane. Colouring is as indicated in the transmembrane helix schematic on the left. b, Through-membrane views, at the same orientation as in a, indicating the TM3 break (red) and the aqueous volumes within the vestibules (blue). c, Bpe with S7 monobodies bound. Variable sequences of the monobodies (cyan) are shown with ribbon or mesh representation. A single-channel Bpe recording showing S7 (500 nM) binding and dissociation is shown below. Zero-current level is indicated by the dashed line.
Abstract
To contend with hazards posed by environmental fluoride, microorganisms export this anion through F(-)-specific ion channels of the Fluc family. Since the recent discovery of Fluc channels, numerous idiosyncratic features of these proteins have been unearthed, including strong selectivity for F(-) over Cl(-) and dual-topology dimeric assembly. To understand the chemical basis for F(-) permeation and how the antiparallel subunits convene to form a F(-)-selective pore, here we solve the crystal structures of two bacterial Fluc homologues in complex with three different monobody inhibitors, with and without F(-) present, to a maximum resolution of 2.1 Å. The structures reveal a surprising ‘double-barrelled’ channel architecture in which two F(-) ion pathways span the membrane, and the dual-topology arrangement includes a centrally coordinated cation, most likely Na(+). F(-) selectivity is proposed to arise from the very narrow pores and an unusual anion coordination that exploits the quadrupolar edges of conserved phenylalanine rings.