A chimeric prokaryotic pentameric ligand-gated channel reveals distinct pathways of activation
By Nicolaus Schmandt, Phanindra Velisetty, Sreevatsa V. Chalamalasetti, Richard A. Stein, Ross Bonner, Lauren Talley, Mark D. Parker, Hassane S Mchaourab, Vivien C. Yee, David T. Lodowski, and Sudha Chakrapani1,
Published in J Gen Physiol. 2015 Oct;146(4):323-40. PMID: 26415570. PMCID: PMC4586589. Link to Pubmed page.
Core Facility: Spectroscopy and Instrumentation Core.
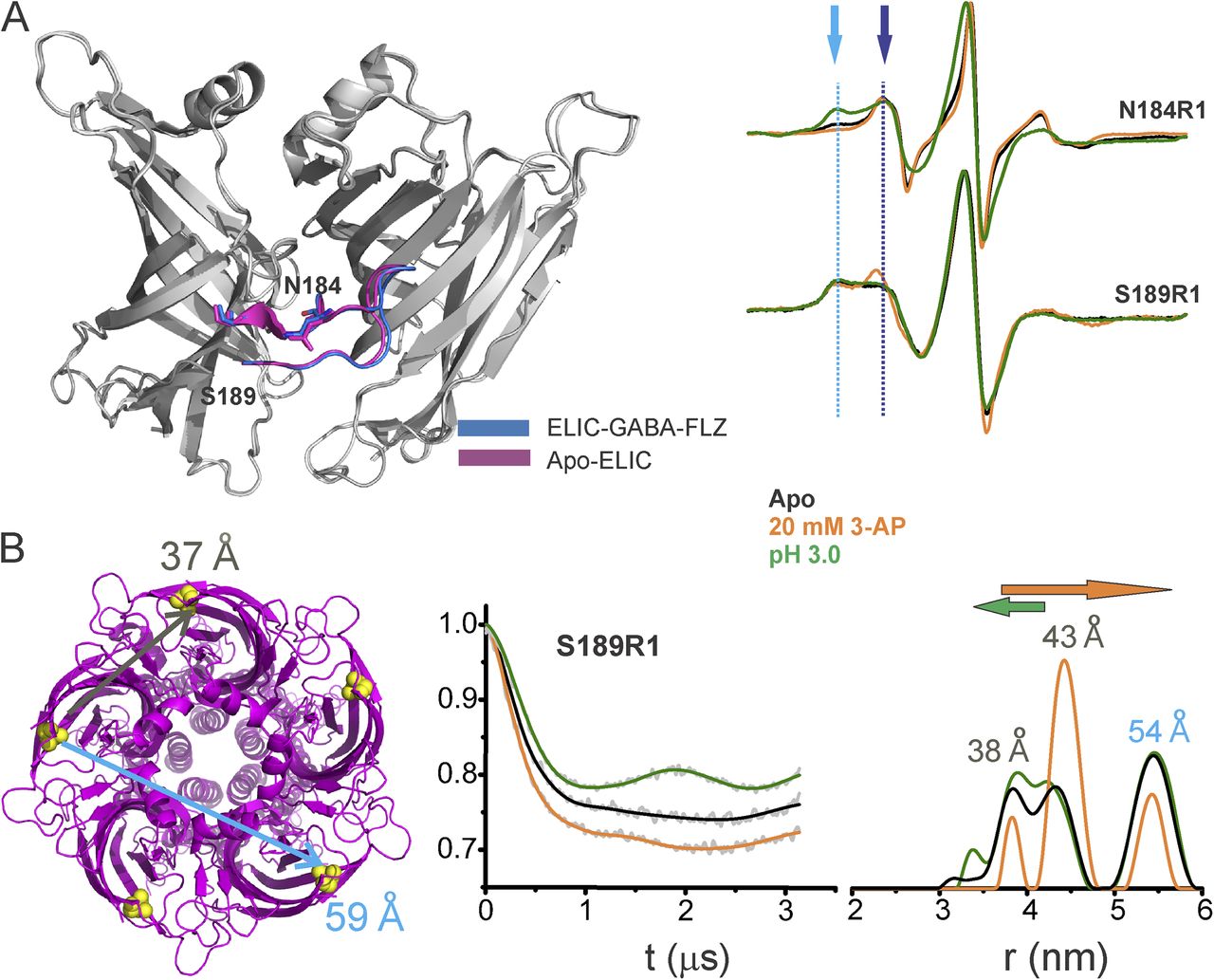
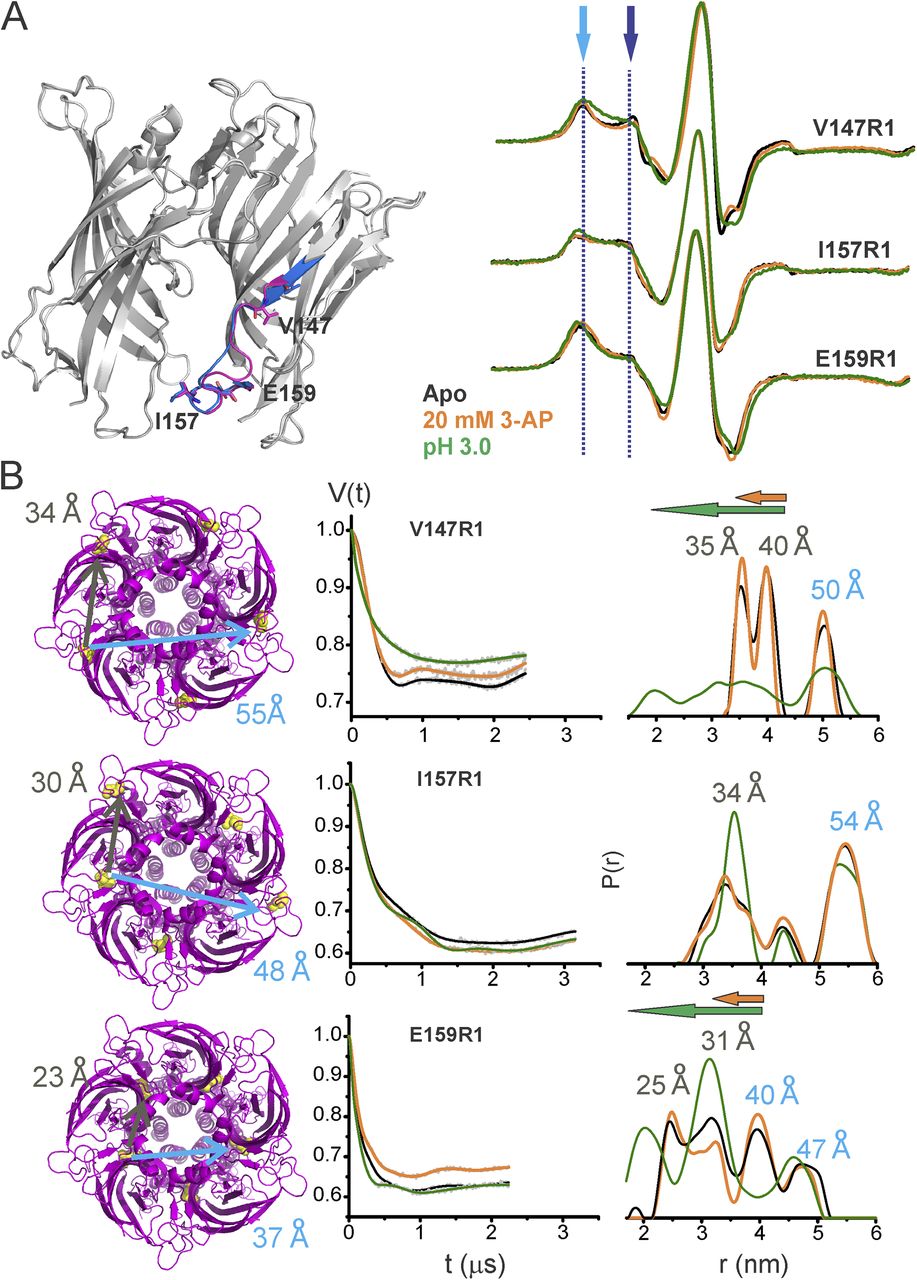
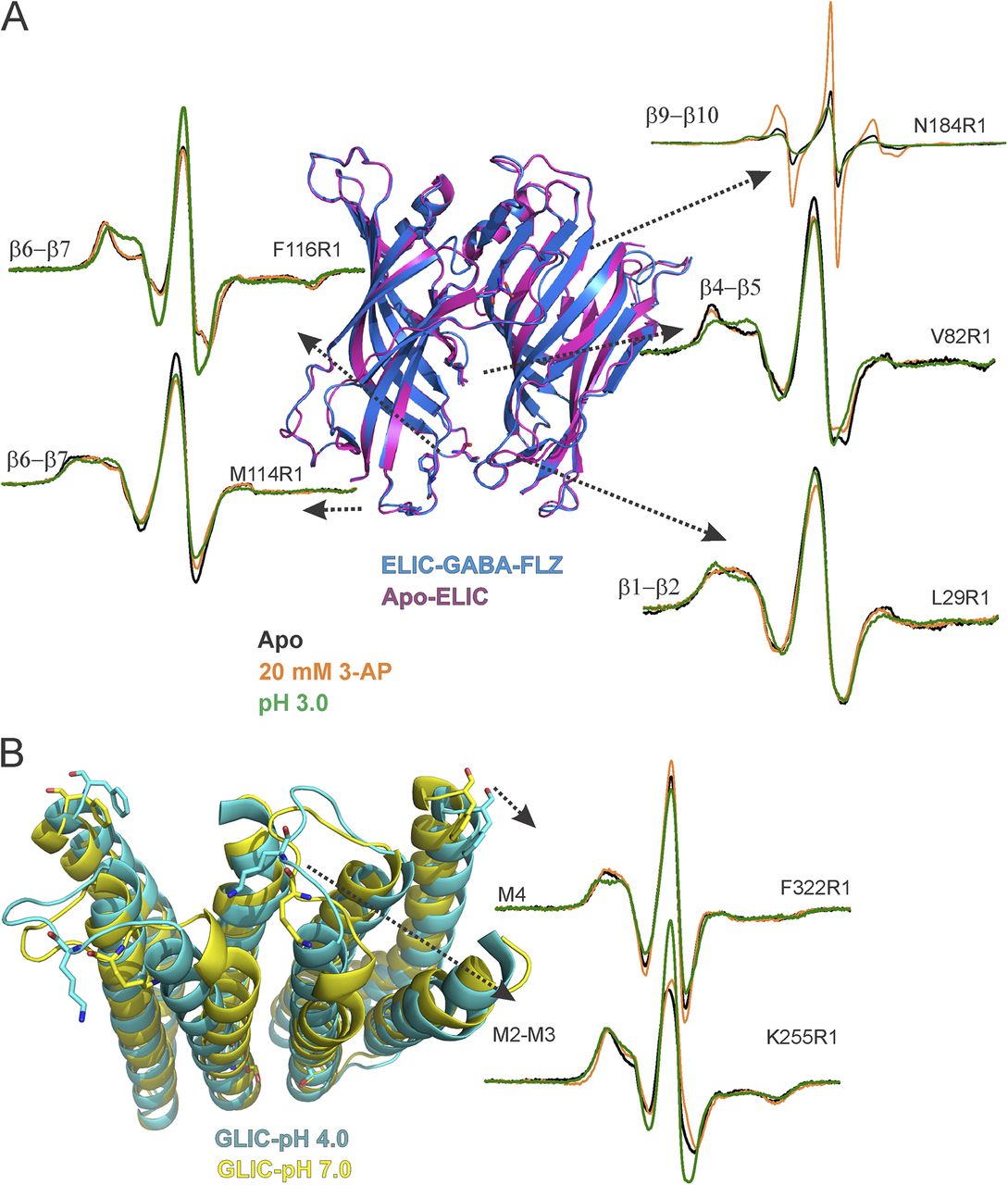
Figure 5. Loop C movements in the ELIC-GLIC chimera. (A) An overlay of ELIC-ECD in the apo (PDB accession no. 3RQU)- and GABA-flurazepam (PDB accession no. 4A96)–bound forms, with residues Asn184 and Ser189 highlighted (left). CW-EPR line shapes for the loop C positions in the apo conformation, and in the presence of 20 mM 3-AP, or at pH 3.0. The light and dark blue arrows highlight the immobile and mobile components of the spectra, respectively. (B) ELIC structure showing the location of Ser189 and corresponding cβ-cβ distances for the adjacent and nonadjacent subunits (left). Background-subtracted DEER echo intensity is plotted against evolution time and fit using model-free Tikhonov regularization. The corresponding interspin distance distribution (right). The arrows highlight the direction of change (orange, 3-AP; green, pH 3.0).
Abstract
Recent high resolution structures of several pentameric ligand-gated ion channels have provided unprecedented details of their molecular architecture. However, the conformational dynamics and structural rearrangements that underlie gating and allosteric modulation remain poorly understood. We used a combination of electrophysiology, double electron-electron resonance (DEER) spectroscopy, and x-ray crystallography to investigate activation mechanisms in a novel functional chimera with the extracellular domain (ECD) of amine-gated Erwinia chrysanthemi ligand-gated ion channel, which is activated by primary amines, and the transmembrane domain of Gloeobacter violaceus ligand-gated ion channel, which is activated by protons. We found that the chimera was independently gated by primary amines and by protons. The crystal structure of the chimera in its resting state, at pH 7.0 and in the absence of primary amines, revealed a closed-pore conformation and an ECD that is twisted with respect to the transmembrane region. Amine- and pH-induced conformational changes measured by DEER spectroscopy showed that the chimera exhibits a dual mode of gating that preserves the distinct conformational changes of the parent channels. Collectively, our findings shed light on both conserved and divergent features of gating mechanisms in this class of channels, and will facilitate the design of better allosteric modulators.