Combining in Vitro Folding with Cell Free Protein Synthesis for Membrane Protein Expression
By Paul J. Focke, Christopher Hein, Beate Hoffmann, Kimberly Matulef, Frank Bernhard, Volker Dötsch, and Francis I. Valiyaveetil.
Published in Biochemistry 2016 Aug 2;55(30):4212-9. PMID: 27384110. PMCID: PMC5021318. Link to publication page.
Core Facility: Membrane Protein Expression/Purification.
Abstract
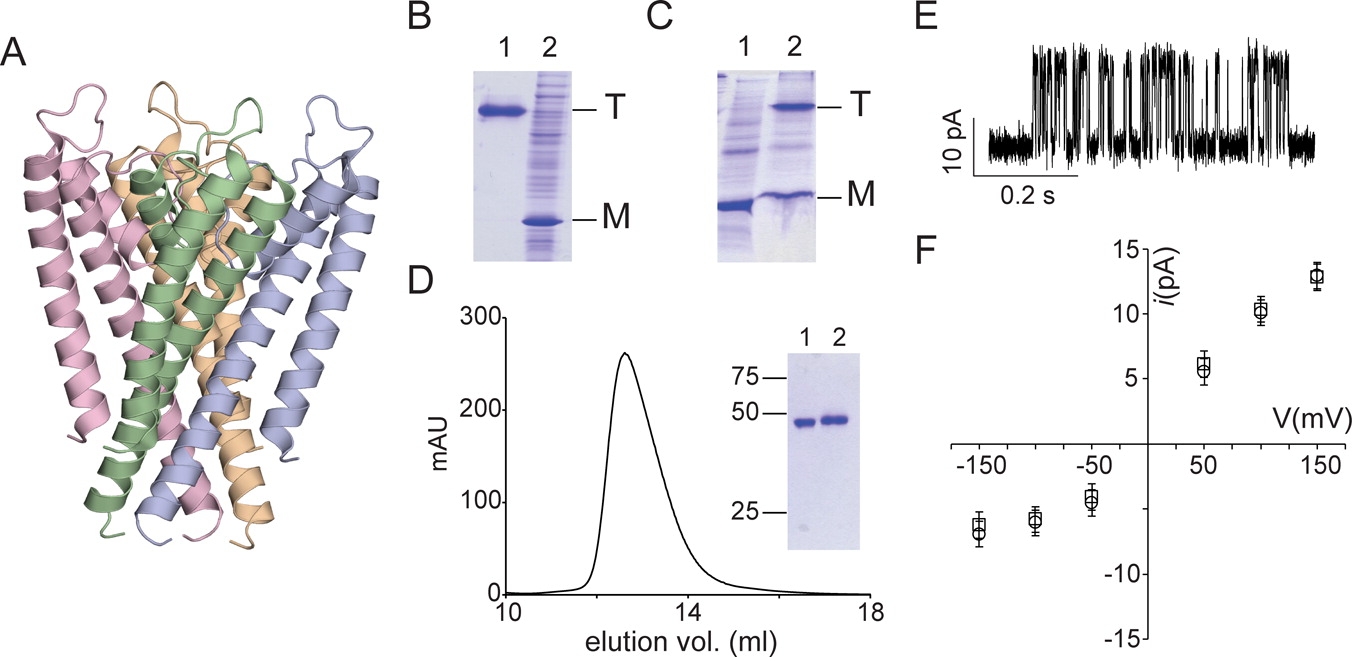
Cell free protein synthesis (CFPS) has emerged as a promising methodology for protein expression. While polypeptide production is very reliable and efficient using CFPS, the correct cotranslational folding of membrane proteins during CFPS is still a challenge. In this contribution, we describe a two-step protocol in which the integral membrane protein is initially expressed by CFPS as a precipitate followed by an in vitro folding procedure using lipid vesicles for converting the protein precipitate to the correctly folded protein. We demonstrate the feasibility of using this approach for the K(+) channels KcsA and MVP and the amino acid transporter LeuT. We determine the crystal structure of the KcsA channel obtained by CFPS and in vitro folding to show the structural similarity to the cellular expressed KcsA channel and to establish the feasibility of using this two-step approach for membrane protein production for structural studies. Our studies show that the correct folding of these membrane proteins with complex topologies can take place in vitro without the involvement of the cellular machinery for membrane protein biogenesis. This indicates that the folding instructions for these complex membrane proteins are contained entirely within the protein sequence.


