A multipoint hydrogen-bond network underlying KcsA C-type inactivation
By Julio F Cordero-Morales, Vishwanath Jogini, Sudha Chakrapani, and Eduardo Perozo
Published in Biophysical journal 100(10): 2387-93 on May 18, 2011.
PMID: 21575572. PMCID: PMC3093550. Link to Pubmed page.
Project: Dynamics of Ion Permeation and Conformational Coupling in – KcsA
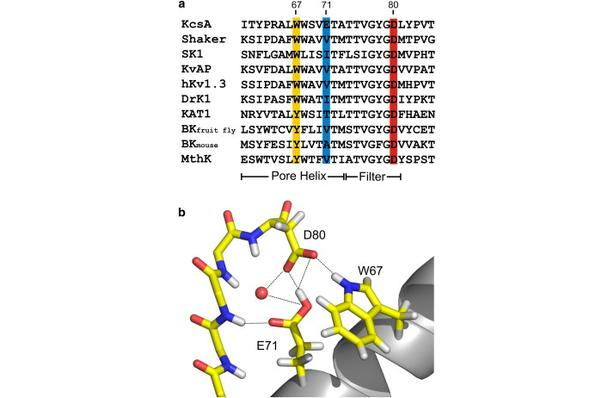
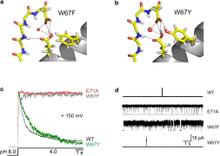
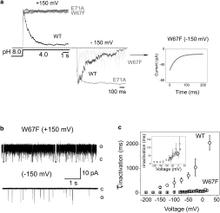
Figure 1. Nature of the hydrogen-bond network surrounding the K+ channel selectivity filter. (a) Sequence alignment of KcsA and a series of Kv channels. We highlight the members of the Glu-71-Asp-80-Trp-67 interacting triad and show their conservation for a variety of Kv channels. (b) High-resolution crystal structure of the KcsA selectivity filter and adjacent structures (1K4C). The Glu-71-Asp-80 and Trp-67-Asp-80 hydrogen-bond interactions between the pore helix and external vestibule are implicated as the driving force for C-type inactivation.
Abstract
In the prokaryotic potassium channel KcsA activation gating at the inner bundle gate is followed by C-type inactivation at the selectivity filter. Entry into the C-type inactivated state has been directly linked to the strength of the H-bond interaction between residues Glu-71 and Asp-80 behind the filter, and is allosterically triggered by the rearrangement of the inner bundle gate. Here, we show that H-bond pairing between residues Trp-67 and Asp-80, conserved in most K⁺ channels, constitutes another critical interaction that determines the rate and extent of KcsA C-type inactivation. Disruption of the equivalent interaction in Shaker (Trp-434-Asp-447) and Kv1.2 (Trp-366-Asp-379) leads also to modulation of the inactivation process, suggesting that these residues also play an analogous role in the inactivation gating of Kv channels. The present results show that in KcsA C-type inactivation gating is governed by a multipoint hydrogen-bond network formed by the triad Trp-67-Glu71-Asp-80. This triad exerts a critical role in the dynamics and conformational stability of the selectivity filter and might serve as a general modulator of selectivity filter gating in other members of the K⁺ channel family.